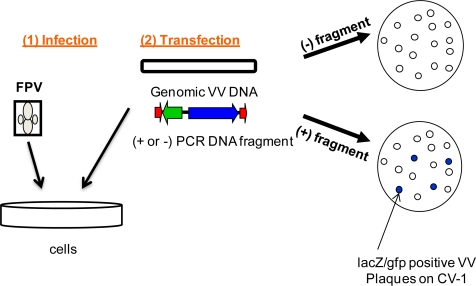
Shortly after the World Health Assembly resolution recommending the cessation of smallpox vaccination, proposals were made to use recombinant vaccinia viruses for immunization against other infectious agents. 161, 162 The idea was to stably insert one or more genes from other pathogens into the vaccinia virus genome while maintaining the latter’s infectivity. Furthermore, the high capacity of the vaccinia virus recombinant for foreign DNA raised the possibility of polyvalent vaccines against multiple diseases.
In principle, recombinant vaccinia viruses would have many of the properties of live attenuated virus vaccines and would naturally present antigens to stimulate humoral immunity to native protein conformation as well as cell-mediated immunity. Such vaccines could also retain the familiar advantages of the smallpox vaccine: heat stability, low cost, ease of administration, and a scar as visible proof of vaccination. Although recombinant vaccinia viruses are still being investigated for human and veterinary vaccination, their great value for vaccine research has been widely recognized.
Purity: greater than 85% as determined by SDS-PAGE.
Destination names: CTSK
Uniprot No.: P18378
Research Area: Others
Alternative names: (K2 protein)
Species: Vaccinia Virus (Western Reserve strain) (VACV) (Vaccinia virus (WR strain))
Source: Baculovirus
Expression Region: 1-88aa
Mole Weight: 11.6 kDa
Protein Length: Total length
Tag Information: N-terminal 6xHis-tagged
Form: Liquid or Lyophilized Powder
Note: We will preferably ship the format we have in stock, however, if you have any special requirements for the format, please remark your requirement when placing the order, we will prepare according to your demand.
Buffer
If the dosage form is liquid, the default storage buffer is Tris/PBS based buffer, 5%-50% glycerol. If the administration form is a lyophilized powder, the buffer before lyophilization is a Tris/PBS-based buffer, 6% trehalose, pH 8.0.
Reconstitution
We recommend that this vial be briefly centrifuged before opening to bring the contents to the bottom. Reconstitute protein in sterile deionized water at a concentration of 0.1-1.0 mg/mL. We recommend adding 5-50% glycerol (final concentration) and an aliquot for long-term storage at -20°C/-80°C. Our final default glycerol concentration is 50%. Customers could use it for reference.
Storage Conditions
Store at -20°C/-80°C upon receipt, need to be aliquoted for multiple uses. Avoid repeated cycles of freezing and thawing.
Shelf life
Shelf life is related to many factors, storage condition, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of the liquid form is 6 months at -20°C/-80°C. The shelf life of the lyophilized form is 12 months at -20°C/-80°C.
Delivery time
The delivery time may differ depending on the way or location of purchase, consult your local distributors for the specific delivery time.
Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
Vaccinia virus as a tool for vaccine research
Recombinant vaccinia viruses provide a powerful means of dissecting the immune responses of humans and experimental animals to individual gene products of infectious agents. Only a few examples can be mentioned here. Therefore, recombinant vaccinia viruses were used to demonstrate that influenza virus HA and NP proteins induced subtype-specific and cross-reactive cytotoxic T-cell responses, respectively. Evidence for human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T cells in patients with acquired immunodeficiency syndrome (AIDS) was first obtained by using recombinant vaccinia viruses expressing envelope or internal proteins to prepare target cells.
Indeed, recombinant vaccinia viruses have become an important tool for cellular immunologists. Because proteins expressed in mammalian cells by recombinant vaccinia viruses are normally folded, processed, and transported, they can be used to induce or bind antibodies that recognize conformational epitopes. The wide host range of vaccinia viruses makes it possible to determine protective immune responses against infectious agents in a variety of experimental animals, from rodents to primates.
For example, the F glycoprotein is more important for inducing protection against respiratory syncytial virus, while the HN protein is better for parainfluenza virus type 3 and type 5. Protection elicited by the virus’s M2 protein syncytial is due to CD8+ T cells, while that induced by F and G proteins is due to antibodies. Similar results, with respect to HA and NP proteins, have been obtained in studies with the influenza virus. In some cases, vaccination has a priming effect followed by an anamnestic antibody response, as suggested for the protection of chimpanzees after inoculation with a recombinant vaccinia virus expressing hepatitis B surface antigen. A list of viruses for which protective immune responses have been elicited can be found in a review.